Losartan Recall List 2022
Losartan Recall List 2022
Irbesartan losartan and valsartan drugs Issue. Food and Drug Administration FDA announced the recall of hundreds of lots of blood pressure drugs. 1170 rows Search List of Recalled Angiotensin II Receptor Blockers ARBs including Valsartan Losartan and Irbesartan. Is recalling 12 lots of prescription irbesartan tablets.

Heart Drug Recall Expanded Again Myfox8 Com
Not treating your condition may pose a greater health risk.
Losartan Recall List 2022. The recall affected six lots of 25 mg strength and 29 lots of 100 mg. Common blood pressure drugs are being recalled by the UK medicine regulator over fears of contamination with a substance that can increase the risk of cancer over time. OTTAWA ON May 30 2021 CNW -.
UPDATE June 15 2021. Several companies are recalling multiple lots of these products due to the presence of an azido. In July 2018 valsartan was the first blood pressure drug recalled.
Irbesartan losartan and valsartan drugs Issue. Irbesartan and Losartan containing products. Recalls 12 lots of irbesartan drugs due to azido impurity Sanofi Consumer Health Inc.
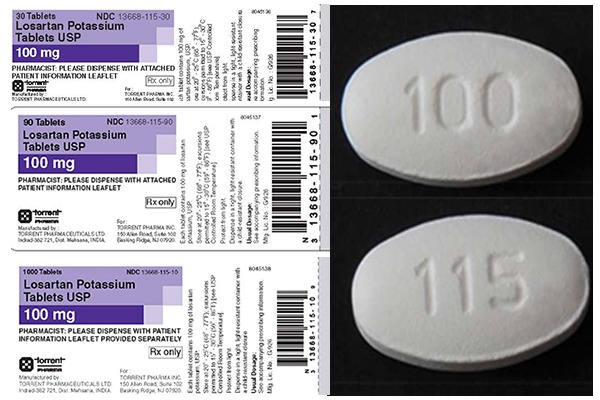
Torrent Recalls Losartan Because Of Carcinogen Long Island Accident Attorneys Rglz Personal Injury Law

Updated Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall Of Losartan Potassium Tablets Usp And Losartan Potassium Hydrochlorothiazide Tablets Usp Fda

Legacy Pharmaceutical Packaging Llc Issues Voluntary Nationwide Recall Of Losartan Potassium Tablets Usp 25mg 50mg And 100mg Due To The Detection Of Trace Amounts Of N Nitroso N Methyl 4 Amino Butyric Acid Nmba Impurity
With Teva And Torrent Recalls Of Tainted Losartan Now Add Up To 368 Lots Fiercepharma

Updated Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall Of Losartan Potassium Tablets Usp And Losartan Potassium Hydrochlorothiazide Tablets Usp Fda

Blood Pressure Medication Losartan Recalled Roman Healthguide
Valsartan Losartan And Other Blood Pressure Medication Recalls 2018 19

Fda Recalls Popular Blood Pressure Med Losartan For Possible Cancer Causing Impurity Manchester Ink Link

Fda Expands Losartan Recall Again

Updated Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall Of Losartan Potassium Tablets Usp And Losartan Potassium Hydrochlorothiazide Tablets Usp Fda

Updated Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall Of Losartan Potassium Tablets Usp And Losartan Potassium Hydrochlorothiazide Tablets Usp Fda

Torrent Pharmaceuticals Recalls Its Blood Pressure Medications

Additional Losartan Drugs Recalled Over Carcinogen Contamination Concerns Drug Safety News
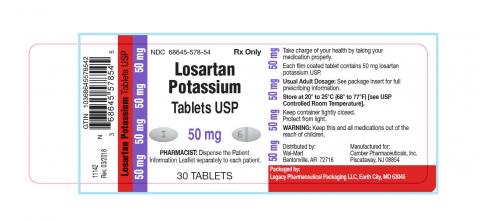
Legacy Pharmaceutical Packaging Llc Issues Voluntary Nationwide Recall Of Losartan Potassium Tablets Usp 25mg 50mg And 100mg Due To The Detection Of Trace Amounts Of N Nitroso N Methyl 4 Amino Butyric Acid Nmba Impurity

More Blood Pressure Medication Recalled For Small Amounts Of Possible Cancer Causing Substance

Blood Pressure Medications Recall

No Need To Raise The Pressure On Losartan The Care Issue

Blood Pressure Medication Recall Expands Again With New Lots Of Losartan Wqad Com
What Are The Lot Numbers Of Losartan Recall What Are The Lot Numbers Of Losartan Recall Fast Shipping
Post a Comment for "Losartan Recall List 2022"